Images
Particle Model of Matter
<div style=”margin-bottom:5px”> <strong> <a href=”https://www.slideshare.net/LKOTZE/particle-model-of-matter-a-quick-summary” title=”Particle model of matter a quick summary” target=”_blank”>Particle model of matter a quick summary</a> </strong> from <strong><a href=”http://www.slideshare.net/LKOTZE” target=”_blank”>Lily Kotze</a></strong> </div>
Please note: transitions and animations are not supported by Slide Share, so should you want to use the slide show you will need to download it from Slide Share.
A few properties of solids, liquids and gases:
Remember, solids like your pencil case, the mug you drink your tea from and your pen you write with all have a fixed shape. Even sugar crystals or salt. The only reason you can pour salt or sugar, is because you have really small clusters of particles. Liquids and gases, on the other hand, are easily poured, because the particles are not fixed in one place: they can move about. Liquids and gases also take the shape of the container – like the jug of juice at the breakfast table.
If you hold on to your pen or space case, it is hard. It doesn’t squish together. You cannot push your finger into your space case lid without breaking it (or your finger). Liquids and gases are again different. If you test how hot your tea is, you push your finger into the tea. Had the liquid been hard, it would feel like pressing your finger into the lid of your space case. If gases were hard, you would not be able to walk, but luckily you move through the gas particles in the atmosphere very easily.
The particles in your space case are close together, with lots of particles together in a small space. This indicates a high density. As you move from solids to liquids to gases, the spaces between the particles in a specific volume of space increases and the amount of particles become less. This means the density decreases.
When you take your ruler, hold it between your index finger and thumb. Now squeeze. Did it squish like a spunge? No. Solids are not compressible. If you collect water in a syringe and close the nozzle of the syringe and press the plunger in, you will feel that the plunger does not move. This is why so many textbooks say that liquids are not compressible. But if you do the same with a gas, the plunger visibly moves, so the particles take up some of the empty space between them, effectively moving closer to each other and is thus compressible.
Diffusion:
When you get home after school, is it not just the best ever to walk in and smell the food on the stove? The smell permeates right through the house, right? But where did that smell start? In the kitchen, of course. So the smell had to move. This happens through the process of diffusion. Particles move from a high concentration (the pot on the stove) to a low concentration (the rest of the house).
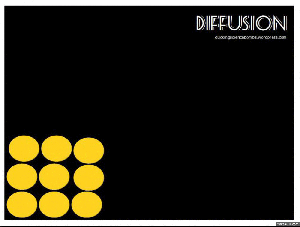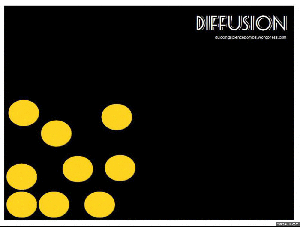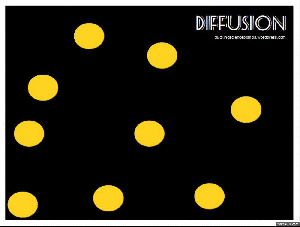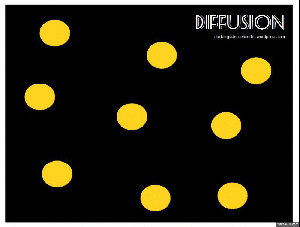
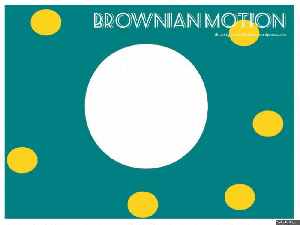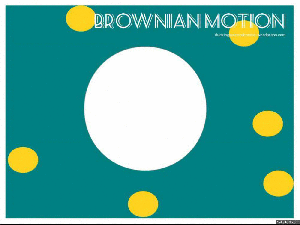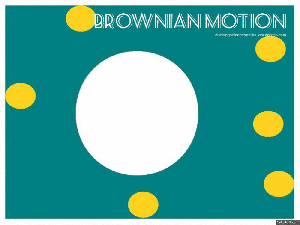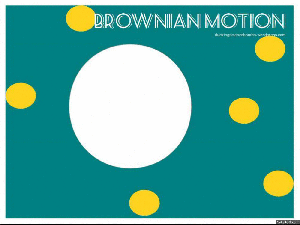
Brownian motion:
Brownian motion is named after Robert Brown – a botanist in the 1800’s, who looked at a bit of pollen on a microscope slide. He saw that the pollen, suspended in water, moved about.
This moving about is what we know as Brownian Motion. The pollen particle (in our GIF it is the big white blob) is bombarded by water molecules (the yellow dots), which then makes the pollen particle move about.
When a substance is heated up, the particles move about more, increasing their individual kinetic energy. When things move more, they cannot stay fixed to other objects, so the particles break apart and move further away from each other. This means that substances move from solids, to liquids to gases. When you cool things down, the particles move slower, making it easy for particles to stick together and change from gas to liquid to solids.
The “sticking together” of particles are known as intermolecular forces, which describes the forces between particles. Between solid particles, you will have stong intermolecular forces, because solid particles like sticking together, in liquids this force is weaker and in gases it is negligible.
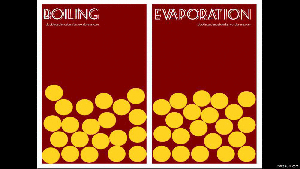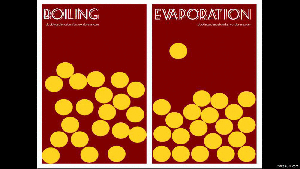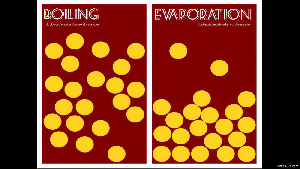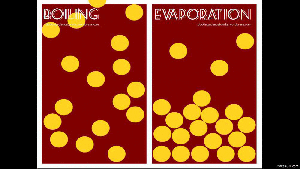
When a liquid changes from a liquid to a gas, you have two ways of doing this: boiling and evaporation.
Remember that boiling is when you add heat (like putting a pot of water on the stove), and evaporation is when the energy from the environment cause the water particles to break away (like a fish pond’s water level dropping).
See some more (and cleverer) sites for extra help:
BBC – KS3 Bitesize Science:

